Abstract
Introduction: While the prognosis of Multiple Myeloma (MM) has dramatically improved in recent years due to the introduction of novel agents and immunotherapies. A group of ~15% of patients, considered to have high-risk disease, do not benefit to the same extent and experience death within 2-3 years from diagnosis. Notably, besides MM progression a major cause of death is non-relapse mortality, mainly due to infectious complications, supporting a link between clonal high-risk features and increased immunosuppression. Vice versa, the main risk factor for non-relapse mortality in MM is high-risk disease (Mai EK et al, 2018), for reasons which has yet to be elucidated. In the era of T-cell based immunotherapies, this unfavorable interaction between cancer cells and components of the immune system becomes even more important. In the KarMMa trial evaluating Idecabtagen-Vicleucel in relapsed refractory (RR)MM, high-risk states defined as R-ISS-3 recorded at enrollment conferred decreased response rates and poor progression free survival. Of note, R-ISS-3 is a composite feature of high-risk fluorescence in situ hybridization and high tumor load, bringing the degree of bone marrow (BM) plasma cell (PC) infiltration into play.
Recently, a number of reports have highlighted that energy metabolites such as lactate or certain amino acids within the microenvironment massively impact tumor immunity, e.g. increased levels of lactate support immune suppressive Treg function, whereas cytotoxic T cells compete for glucose and glutamine with tumor cells, altering their functionality. Our hypothesis is that specific metabolic changes underlie the unfavorable interplay between high-risk disease and immunosuppression.
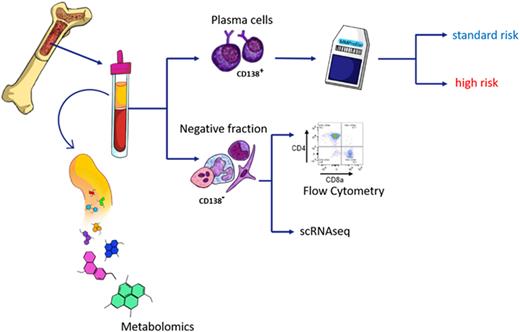
Methods: We assessed MM risk status in 231 patients using the gene expression profiling based SKY92 micro-array platform, which assigns patients to high and standard risk. To investigate the interplay between tumor cells and immune cells, we collected CD138 negative BM fractions and processed them by flow cytometry and for selected cases by single cell RNA sequencing. Furthermore, we collected bone marrow plasma from the same samples for liquid chromatography mass-spectrometry (LC/MS).
Results: Our flow cytometry analysis showed profound differences in the T cell composition between high-risk and standard-risk patients. Overall, high-risk patients displayed lower numbers of T cells, which was mainly attributed to lower frequencies of CD4 positive T cells. Hence, the CD8/CD4 ratio was significantly increased in high-risk patients when compared to standard-risk (P< 0.0001). We went on and investigated the metabolic phenotype of T cells using metabolic markers such as 2-NBDG (fluorescent indicator of direct glucose uptake) and BODIPY (neutral lipids staining) by flow cytometry. Both markers were significantly increased in CD4 positive T cells in high-risk patients (P<0.01 and P<0.04 for 2-NBDG and BODIPY, respectively). Our results support the notion that a distinct metabolic program underlies aberrant CD4 frequencies in high-risk disease.
To further investigate the metabolic mechanisms shaping the immune cell composition and function in the BM, we compared water-soluble metabolites from BM plasma between high-risk and standard-risk patients. High-risk patients showed an increase in plasma concentration of nicotinamide and decreased glutamine levels when compared to standard-risk. Previously, it was shown that nicotinamide decreases cytotoxic T cell function in vitro (Agliano F et al, 2022). Additionally, we observed major differences when comparing high BMPC infiltration versus low BMPC infiltration (cut off 30%), including a number of amino acids and their degradation products such as glutamine and taurine, which were significantly depleted in patients with high BMPC infiltration.
Conclusions: Our study highlights an additional characteristic of high-risk disease, which is not solely related to genomic changes of tumor cells rather than by profound differences in T cell frequencies and functions. Our study supports metabolic changes in the BM micro-environment to drive or shape these pathological processes.
Disclosures
Einsele:BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants; Sanofi: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Other: travel grants. Rasche:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.